Note-A-Rific: Photoelectric Effect
Hertz Experiment
In 1887 (about a decade before Planck did all his stuff to explain blackbody radiation) Hertz was trying to figure out a way to test Maxwell’s theories on electromagnetism.
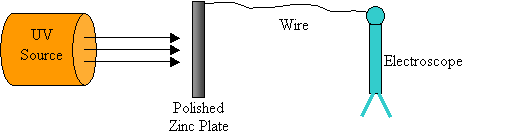
He set up an apparatus as shown here…
|
|
|
|
|
|
While trying to make EM waves, he noticed that when certain metals are hit by ultraviolet (UV) radiation, the leaves of the electroscope spread apart.
· He theorized that when UV radiation hit the zinc plate it must liberate (set free) electrons.
· This became known as the “Photoelectric Effect”
photo ŕ light
electric ŕ electricity
· Hertz didn’t develop this discovery much further.
This didn’t disagree with the wave theory of light, since the electric field of an EM wave could be exerting a force on the electrons, forcing them out.
Einstein Investigates Photoelectric Effect
At the beginning of the 1900’s Albert Einstein linked Hertz’ research on the photoelectric effect to the work of Planck.
· Remember, Planck said that an object emits energy according to the formula E = nh¦.
· Einstein said that when an object emits light, the object must decrease its energy by that same amount… E= nh¦.
· So light must be released in “packets” of energy…
· Wait a second… he saying light is emitted as tiny particles, not waves…
· He called these packets of light “photons”
To test his theory, Einstein examined the photoelectric effect.
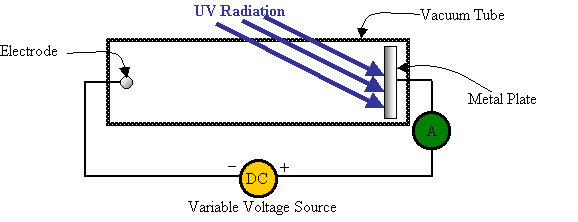
A device similar to this one was set up:
|
|
|
|
|
|
Einstein ran several different tests with this apparatus.
1. What happens when the voltage source is turned off and the device is in the dark (no UV radiation falls on the plate)?
Nothing happens. There are no readings on the ammeter, which is exactly what we would expect. As shown above, this is a broken circuit that (at the moment) has no voltage source.
2. What happens when the voltage source is turned off and the device is exposed to radiation with a frequency less than UV?
Again, nothing happens. This agrees with the original experiment that Hertz performed, since he found that you need frequencies of radiation that were equal or greater than UV before anything happens at the metal plate.
3. What happens when the voltage source is turned off and the device is exposed to radiation with a frequency equal to or greater than UV?
Now a current is shown by the ammeter readings! Einstein hypothesized that there were electrons being “ripped” off of the metal plate (as Hertz had observed). Einstein believed that these electrons then moved towards the electrode and hit it, which completes the circuit. This is why a current is shown on the ammeter.
4. What happens if the voltage source is turned on, and slowly increased?
Notice that the variable voltage source is set up so that the electrode will be negative and the metal plate becomes positive. This voltage should work against the electrons getting all the way from the metal plate to the electrode. Only electrons with sufficient kinetic energy (going fast enough) will be able to get to the electrode.
The voltage was slowly increased from zero, and for a while nothing appeared to be changing. But, there came a point when the voltage became too great for electrons to get across the gap. At this point (and for any higher voltages) the ammeter gives a reading of zero.
Stopping Voltage
The minimum voltage needed to stop the electrons from getting across the gap, stopping the current, is called the Stopping Potential č Vstop
· The maximum kinetic energy that the electrons had can the be found from…
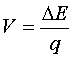
Since the electrons that are going the fastest just barely don’t make it across when the voltage is set at Vstop, the electrons must be losing all of their kinetic energy going across that gap. That’s the change in energy (ΔE) in the formula.
Therefore, the most energy the electrons could have to begin with is found by solving the formula above to get…
Ek max= q Vstop
Example: What is the maximum kinetic energy of electrons emitted from a zinc surface if they are stopped by a 16 N/C uniform electric field over a distance of 3.0cm?
First calculate the voltage…E = V/d V = E d = (16N/C) (0.030m) V = 0.48V |
Second, calculate the maximum kinetic energy… Ek max= q Vstop = (1.6 x 10-19C) (0.48V) Ek max= 7.68 x 10-20 J |
Work Function
Einstein believed that to give a single electron this energy to move, a single photon hit the metal surface (destroying itself), and transferred its energy to the one electron.
· Since the electron is attracted to the surface of the metal, some minimum amount of energy must be needed just to snap it off. Otherwise, electrons would just be dropping off of atoms all the time.
· Einstein called this the work function of the material, since you needed to do work on the electron to break it off.
· Since yanking the electrons started to happen at a minimum frequency (usually around UV), he called it the threshold frequency of the material.
· This was all related to Planck’s formula E=hf in the following way…
|
|
|
|
|
|
|
W = h ¦o
· The work function of materials goes as high as about 10eV.
Example: What is threshold frequency of a material with a work function of 10eV
Since the value for the work function is given in electron volts, we might as well use the value for Planck’s constant that is in eV s.
W = h ¦o
¦o = W / h
= (10eV) / (4.14 x 10-15eVs)
¦o = 4.14 x 10-14 Hz
Photoelectric Effect Formula (the Biggy!)
· If the frequency of the incoming light is great enough, there should be enough energy to break off the electron and have some left over to give it some kinetic energy. So…
h ¦ = Ek max + W
· Which just basically says, if a photon (E=h¦) transfers its energy to an electron, the electron has energy to tear away from its surface (W) and energy to move (Ek)
· This follows the conservation of energy, since the photon’s original energy is equal to the energy it takes to snap off the electron and get it moving.
· Note: some electrons will need more than the bare minimum W to be released (they might be attracted more strongly), so their Ek is not as great as the maximum.
· That’s ok, though, since we’ll only worry about the electrons that came off the easiest and have the maximum kinetic energy.
Example: The work function of silver is 4.73eV. EM radiation with a wavelength of 1.20x1015Hz strikes a piece of pure silver. What is the speed of the electrons that are emitted?
To figure out the answer you will need to go through a few steps…
|
Calculate the maximum Ek first… hf = Ek + W Ek = hf – W = (4.14x10-15eVs)(1.20x1015Hz) – 4.73eV Ek = 0.238 eV = 3.81 x 10-20J |
Then calculate the speed of the electron… Ek = ˝ mv2
v = 2.89 x 105m/s |
Notice that in this example I used the value for Planck’s constant that is given in eVs, rather than Js. This saved me the trouble of changing the work function into Joules. But, it is just as important to realize that this results in an answer in eV which I have to change into Joules before I can use the kinetic energy formula. If you are more comfortable always working with standard units, go for it! If you changed the work function for silver into Joules you’d be doing great. In fact, if you’re ever in doubt, change everything into standard units and go from there. Because eV are so common in this unit.